Published in Nature Communications Physics, a new study by Loughborough University and Nottingham Trent University reveals how these microorganisms transition from random, disorganised states to interwoven patterns that form ‘biomats’, uncovering the fundamental principles behind this natural phenomenon.
This feature explores the capabilities of these brilliant bacteria and how researchers unravelled one of nature’s oldest mysteries.
Why study cyanobacteria
Cyanobacteria are an ancient group of microorganisms with fossils dating back to two billion years ago. They were the first life forms on Earth capable of photosynthesising, contributing to the creation of the planet’s oxygen-rich atmosphere, which made complex life possible.
Today, cyanobacteria remain one of the largest and most significant groups of bacteria, playing a vital role in maintaining the balance of our atmosphere and oceans. They thrive in virtually all aquatic environments across the planet, including frozen Antarctic lakes.
“Cyanobacteria are ubiquitous in any body of water - sea, lakes, rivers”, explains Dr Marco Mazza, an expert in non-equilibrium physics at Loughborough University.
“They produce the lion's share of the oxygen that we breathe and form one of the bases of the food chain in the ocean.
“Understanding how they build their biomats can help us learn about their role in a variety of ecosystems, provide clues about ancient environments where life first evolved, and also inspire new materials for human use.”
What are biomats?
A single cyanobacterium is incredibly small, typically ranging in size from 1 to 30 micrometres (µm) in diameter, depending on the species. To put this into perspective, the thickness of a human hair ranges from about 50µm to 70µm, meaning that numerous cyanobacteria could fit side by side across its width.
Though tiny, cyanobacteria can form colonies called 'biomats', which are visible to the naked eye. These biomats often appear as slippery green slime, commonly referred to as 'blue-green algae', and can be found in stagnant water, riverbeds, and along seashores.
Biomats form when cyanobacteria link together to create thread-like filaments. As their numbers grow, these filaments, capable of moving across surfaces, weave together into intricate patterns that build the biomat structure.

Cyanobacteria filaments under a microscope. Image courtesy of Nottingham Trent University.
Remarkably, a biomat the size of an A4 sheet can appear within hours or days and may contain hundreds of millions of cyanobacteria.

Image: Blooming ‘blue-green algae’ (cyanobacteria).
“Essentially, cyanobacteria invented weaving and knitting before humans”, said Dr Mazza.
“And, as is often the case, we can learn new tricks from the natural world. Imagine if we could create smart clothing or responsive materials that adapt to changes in the external conditions.
“But first we need to understand how cyanobacteria arrange themselves into biomats – a mystery we are now starting to unravel.”
Unravelling cyanobacteria’s biomats
Loughborough University and Nottingham Trent University researchers have been working together to try and understand how cyanobacteria filaments arrange themselves into the patterns seen in biomats.
Using advanced microscopy techniques, simulations and theoretical models, the team previously revealed that when cyanobacteria are present at a high enough density, they begin to organise themselves into patterns by following a few simple rules.
The researchers developed a model to map this behaviour and their new study compares the model's predictions to real-world experiments.
The team cultivated a type of cyanobacteria called Oscillatoria lutea and, using a high-resolution laser scanning microscope, captured how the filaments interact and self-organise at varying densities, and how a biomat develops over time.
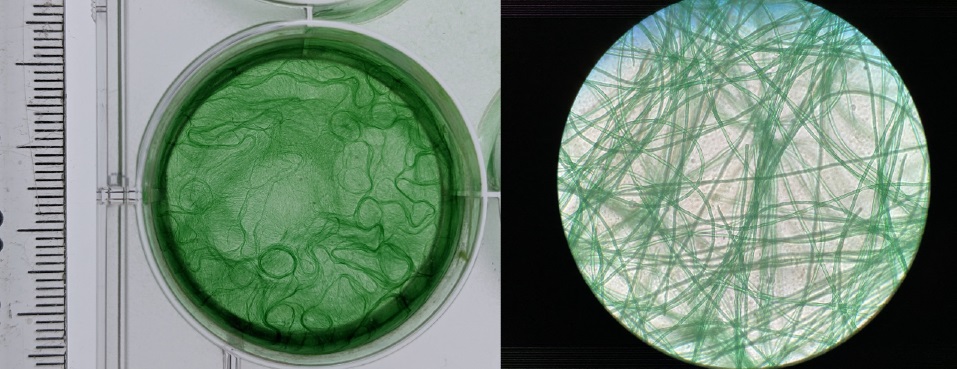
Left: Cyanobacterium cultivated in a laboratory setting. Right: Filaments under the microscope. Image courtesy of Nottingham Trent University.
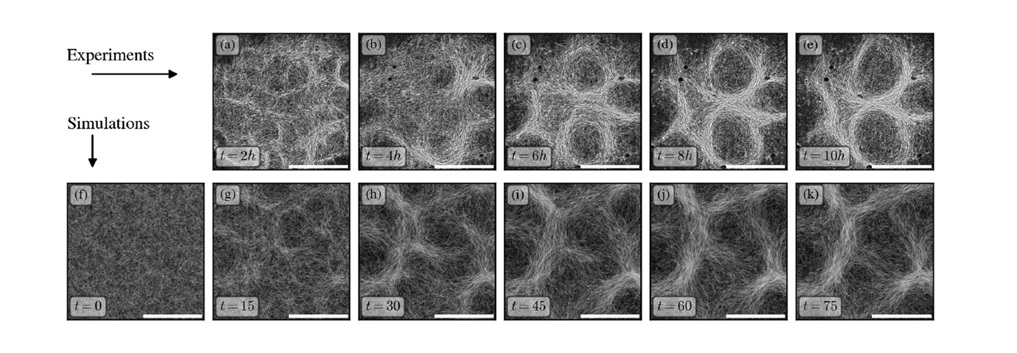
Image: Time-lapse images show how Oscillatoria lutea filaments self-organise into intricate patterns. Panels (a–e) are microscope images of real cyanobacteria, while panels (f–k) are model simulations replicating the same conditions. Both experiments and simulations demonstrate how small features emerge and gradually form stable patterns over a few hours. Original figure caption at: https://doi.org/10.1038/s42005-024-01866-5
The research also reveals that cyanobacteria filament density and motility (ability to move) play a crucial role in pattern formation, with the most organised and stable patterns emerging when filament density and motility are high.
Filament length also plays a role: longer filaments tend to form more aligned, ordered patterns, while shorter filaments create denser but less organised arrangements.
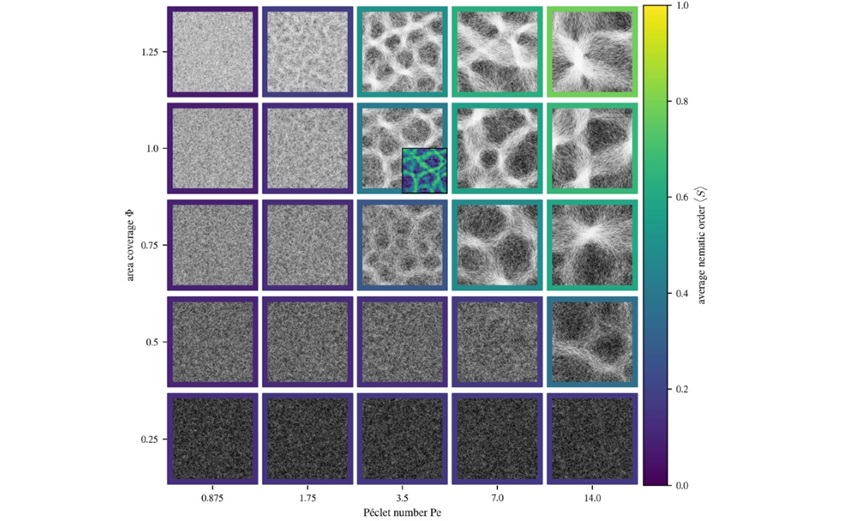
Image: The mosaic of images illustrates how cyanobacteria filaments form stable patterns under different conditions. Each panel represents a simulation and they show how filament arrangement changes with density (area coverage, Φ) and motility (Péclet number, Pe). Original figure caption at: https://doi.org/10.1038/s42005-024-01866-5
The importance of the research
Professor Lucas Goehring, who led the experimental work at Nottingham Trent University, said of the research: “This work shows how the remarkably complex behaviours of biomats, the Earth’s earliest expressions of multicellular life, can be explained by a few simple rules.
“What I found surprising is that the same rules that explain the patterns in mature biomats can also explain, without any significant adjustment, how these patterns grow up.
“The timescales and features that emerge from the model can immediately explain the first few hours of a biofilm colony’s life, as well as its ultimate shape and structure.”
When asked about the importance of the findings, study lead Dr Jan Cammann, a Research Associate in Mathematics at Loughborough University, said: “Now that we understand the mechanisms behind cyanobacteria's organisation into complex biomats, we can start to explore how cyanobacteria biomats respond to physical and chemical changes in their environment.
“Understanding this can help us understand how biomats stabilise sediments in coastal regions and understand how we can prevent biomats forming on ships and other marine structures.
“Ultimately, we hope to further the understanding of one of the most crucial members of the ecosystem whose ability to shape their environment is astonishing.”
The team say that the findings could also help guide future research into how different types of bacteria organise themselves to form structures.
“It could improve our understanding of how bacterial biofilms – collections of bacteria that have attached to a surface and each other – are formed", says Dr Cammann.
“This knowledge is critical given the central role of biofilms in various processes, such as human infections, environmental degradation, and bioengineering.”
The Nature Communications Physics paper, titled ‘Topological transition in filamentous cyanobacteria: from motion to structure’, can be read in full online.
Further information on the Loughborough University and Nottingham Trent University cyanobacteria research can be found in this video: